
CMS released its FY 2022 proposed rules for hospice. Review a breakdown of payment, methodology, and economic impact.
Key insights
- CMS proposes hospice payment update percentage of 2.3% and changes in hospice payment rates for routine home care, continuous home care, inpatient respite care, and general inpatient care.
- CMS discusses its analysis and methodology for reporting with consideration to reporting impacts as a result of the COVID-19 public health emergency.
- The overall economic impact of this proposed rule is estimated to be $530 million in increased payments to hospices for FY 2022.
Have questions about the proposed rules?
The Centers for Medicare & Medicaid Services (CMS) released proposed rules to update the hospice wage index, payment rates, and aggregate cap amount for fiscal year (FY) 2022. Additionally, this rule proposes to make permanent selected regulatory blanket waivers that were issued to Medicare-participating hospice agencies during the COVID-19 public health emergency and update the hospice conditions of participation. Download the proposed rules from the Federal Register.
Hospice utilization and spending patterns
The proposed rule includes data analysis on historical utilization trends. CMS provides analysis as it relates to hospice utilization such as Medicare spending, utilization by level of care, lengths of stay, live discharge rates, and skilled visits during the last days of life using the most recent, complete claims data.
FY 2022 labor share
CMS proposes to rebase and revise the labor shares for continuous home care (CHC), routine home care (RHC), inpatient respite care (IRC), and general inpatient care (GIP) using 2018 Medicare cost report (MCR) data for freestanding hospice facilities. Table 11 (reproduced below) provides the proposed labor share for each level of care based on the compensation cost weights CMS derived using its proposed methodology.
TABLE 11: Proposed and Current Labor Shares by Level of Care

Proposed routine FY 2022 hospice wage index and rate update
This rule proposes updates to the hospice wage index, discussion for the proposed FY 2022 hospice payment update percentage of 2.3%, updates to the payment rates, and updates to the hospice cap amount for FY 2022 by the hospice payment update percentage of 2.3%.
1. Proposed FY 2022 hospice wage index — For FY 2022, the proposed hospice wage index would be based on the FY 2022 hospital cost reporting periods beginning on or after October 1, 2017 and before October 1, 2018 (FY 2018 cost report data). The proposed FY 2022 hospice wage index would not include a cap on wage index decreases and would not take into account any geographic reclassification of hospitals.
2. Proposed FY 2022 hospice payment update percentage — The proposed hospice payment update percentage for FY 2022 is based on the current estimate of the proposed inpatient hospital market basket update of 2.5%, reduced by a multifactor productivity (MFP) adjustment (currently estimated to be .2 percentage points of FY 2022), for an effective proposed rate of 2.3%.
3. Proposed FY 2022 hospice payment rates — Table 12 (reproduced below) reflects the proposed FY 2022 payment rates for RHC. Table 13 (reproduced below) reflects the proposed FY 2022 payment rates for CHC, IRC, and GIP. The proposed FY 2022 rates for hospices that do not submit the required quality data would be updated by the proposed FY 2022 hospice payment update percentage of 2.3% minus 2 percentage points. These rates are shown in Tables 14 and 15 (reproduced below).
TABLE 12: Proposed FY 2022 Hospice RHC Payment Rates
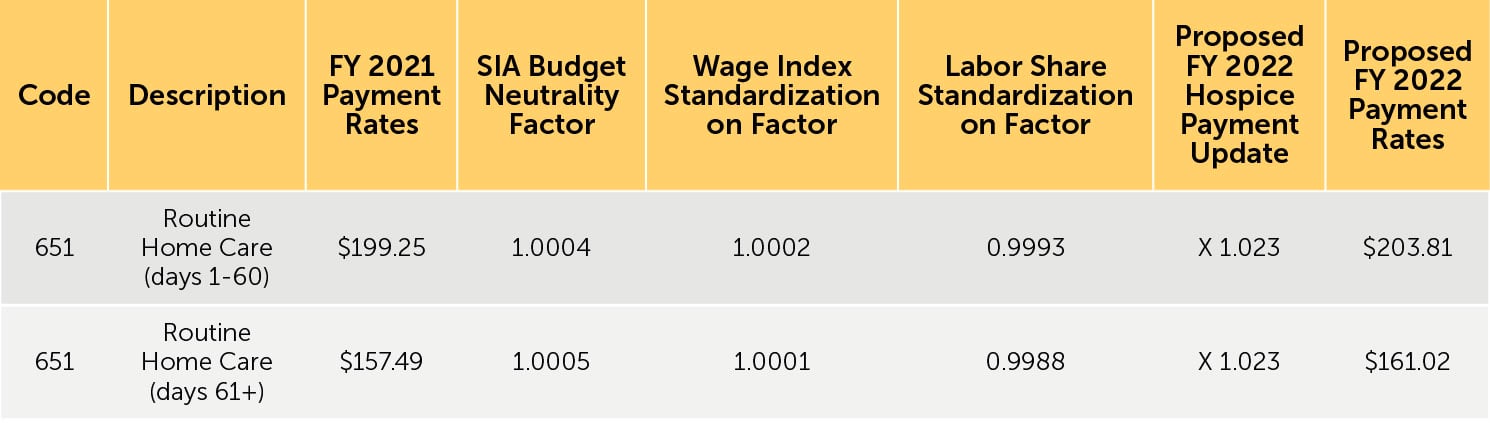
TABLE 13: Proposed FY 2022 Hospice CHC, IPR, and GIP Payment Rates
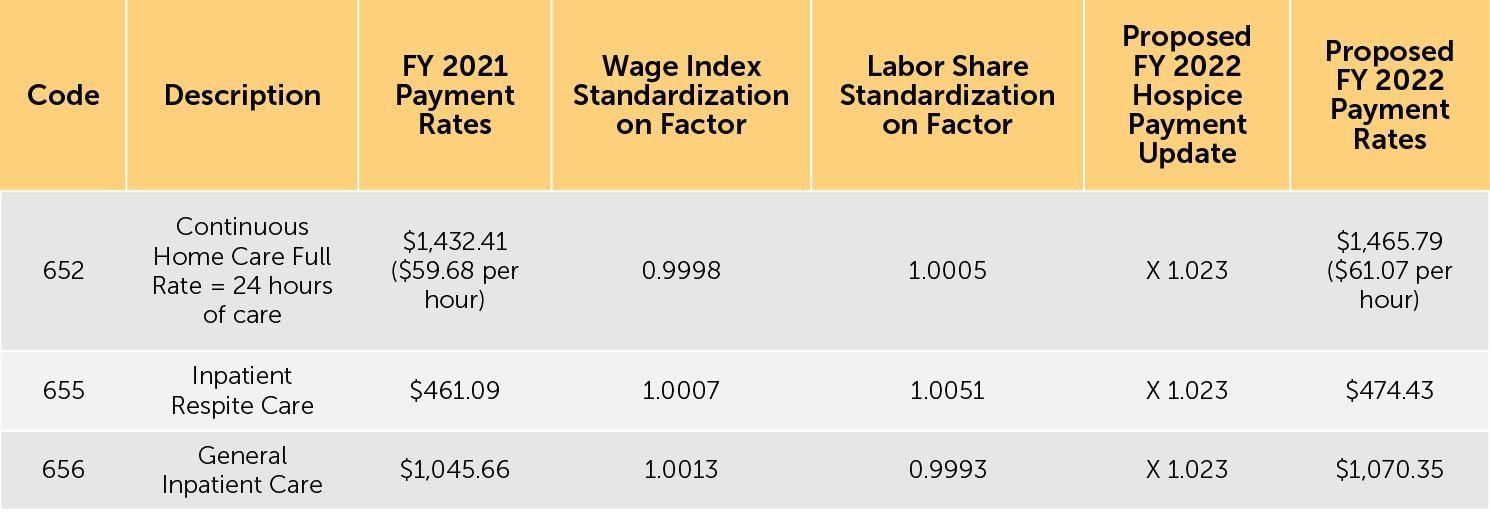
TABLE 14: Proposed FY 2022 Hospice RHC Payment Rates for Hospices That DO NOT Submit the Required Quality Data
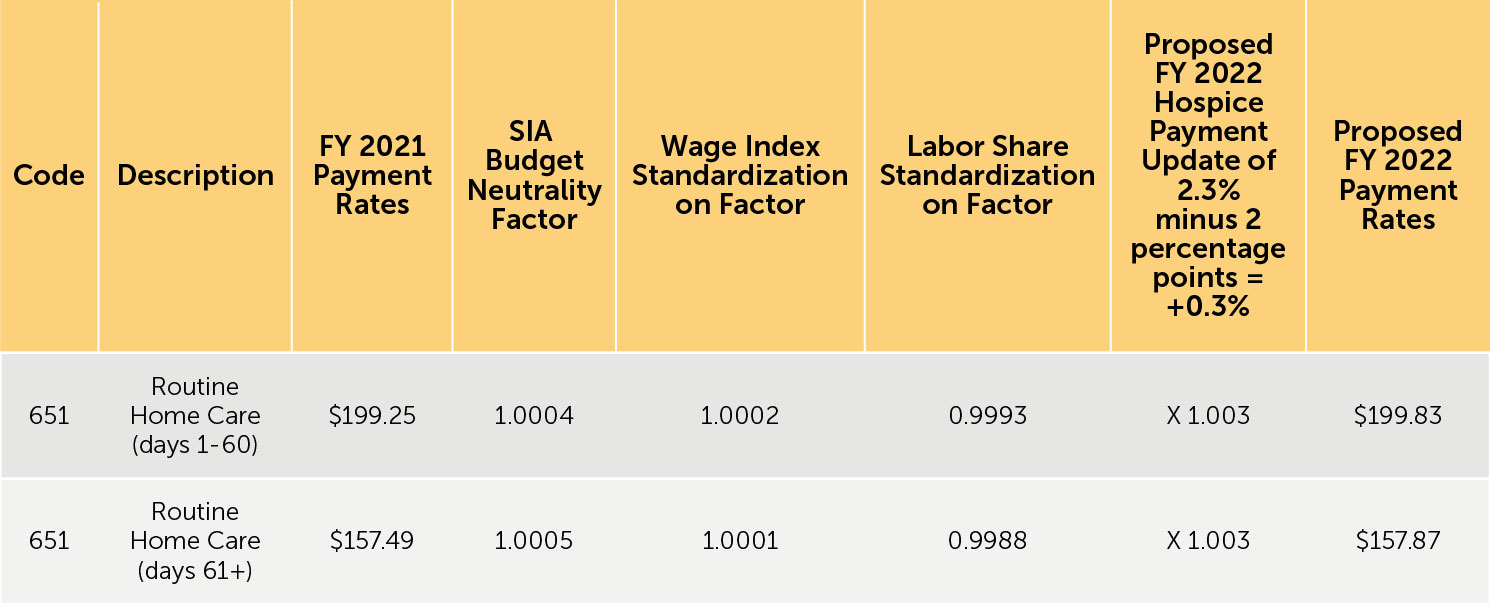
TABLE 15: Proposed FY 2022 Hospice CHC, IRC, and GIP Payment Rates for Hospices That DO NOT Submit the Required Quality Data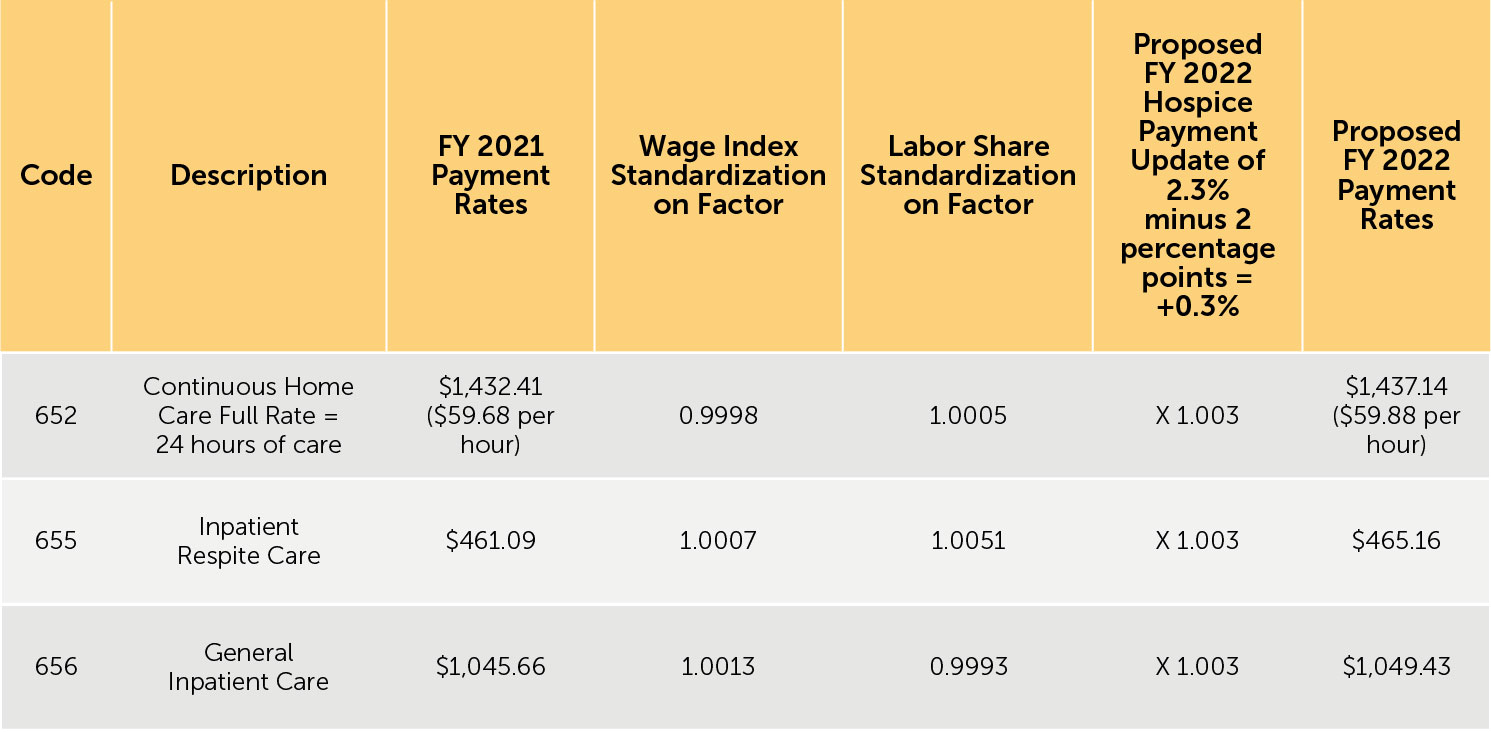
4. Proposed Hospice Cap Amount for FY 2022 — The proposed hospice cap amount for the FY 2022 cap year will be $31,389.66, which is equal to the FY 2021 cap amount ($30,683.93) updated by the proposed FY 2022 hospice payment update percentage of 2.3%.
Proposed clarifying regulation text changes for the hospice election statement addendum
CMS proposes clarifying regulations text changes regarding the election statement addendum requirements that were finalized in the FY 2020 Hospice Wage Index and Rate Update final rule. As this was put into place in 2020, CMS has received stakeholder requests for additional insights.
Hospice waivers made permanent conditions of participation
This rule proposes to make permanent selected regulation blanker waivers and proposes to revise hospice aide requirements to allow the use of the pseudo-patient for conduction hospice aide competency evaluations. Thus, CMS is proposing to permit skill competencies to be assessed by observing an aide performing the skill with either a patient or a pseudo-patient as part of a simulation.
CMS defines a pseudo-patient as a person trained to participate in a role-play situation, or a computer-based mannequin device. CMS defines simulation as a training and assessment technique that mimics the reality of the home care environment.
CMS also proposes to specify that if, during an on-site visit, a hospice verifies the finding of a supervising nurse regarding an area of concern in the performance of a hospice aide, the hospice must conduct and the hospice aide must complete a competency evaluation related to the deficient and related skill(s).
Proposals and update to the Hospice Quality Reporting Program
CMS discusses proposals to the Hospice Quality Review Program (HQRP), updates on the public reporting change for one refresh cycle to report less than the standard quarters of data due to the COVID-19 Public Health Emergency (PHE) exemptions, and adding the Consumer Assessment of Healthcare Providers Systems (CAHPS) Hospice Survey Star ratings.
1. Proposal to remove the seven hospice item set process measures from HQRP beginning FY 2022 — Proposes the removal of the seven Hospice Item Set (HIS) measures from HQRP. CMS determined that the NQF #3235 HIS Comprehensive Assessment Measure is a more broadly applicable measure and continues to provide, in a single measure, meaningful differences between hospices regarding overall quality in addressing the physical, psychosocial, and spiritual factors of hospice care upon admission. Although the proposal removes the seven individual HIS process measures, it does not propose any changes to the requirement to submit the HIS admission assessment.
2. Proposal to add a claims-based index measure, the Hospice Care Index — Proposes a new hospice quality measure, called the Hospice Care Index (HCI), which will provide more information to better reflect several processes of care during a hospice stay, and better empower patients and family caregivers to make informed health care decisions. The HCI is a single measure comprising ten indicators calculated from Medicare claims data. Indicators reflect practices or outcomes hospices should pursue, thereby awarding points based on the criterion.
3. Update the Hospice Visits in the Last Days of Life (HVLDL) and Hospice Item Set V3.00 — CMS discusses the process it used for replacing the Hospice Visits When Death is Imminent II (HVWDII) measure pair (Section O of the HIS V2.01) with the HVLDL measure. OMB approved this replacement from the discharge assessment and HIS V3.00 became effective on Feb 16, 2021 (OMB control number 0938-1153).
4. Proposal to revise submission of hospice quality reporting program data — CMS proposes to revise the regulation by adding paragraphs that would include the existing language on the standardized set of admission and discharge items, would require collection of administrative data, and would be a technical correction to address efforts previously identified.
5. Update regarding the Hospice Outcomes & Patient Evaluation (HOPE) development — The HOPE tool is intended to help hospices better understand care needs throughout the patient’s dying process and contribute to the patient’s plan of care. The two primary objects of HOPE are to provide quality data for the HQRP requirements through standardized data collection, and to provide additional clinical data that could inform future payment refinements. CMA anticipates that HOPE will replace HIS. HOPE will include key items from HIS along with Standardized Patient Assessment Data Elements (SPADEs), and demographics like gender and race.
6. Update on quality measure development for future years — CMS discusses continued updates for both HOPE-based and claims-based quality measure development, including convening a technical expert panel to provide feedback.
7. CAHPS hospice survey participation requirements for the FY 2020 APU and subsequent years — The CAHPS hospice survey is a component of the CMS HQRP used to collect data on the experiences of hospice patients and the primary caregivers listed in their hospice records.
a. Public Reporting of CAHPS Hospice Survey Results — CMS proposes public reporting to continue to be the most recent eight quarters of data, excluding exempted quarters (Quarter 1 and Quarter 2 of CY 2020) as a result of the COVID-19 Public Health Emergency.
b. Volume-based exemption for CAHPS hospice survey data collection and reporting requirements — No changes were proposed to this exemption.
c. Newness exemption for CAHPS Hospice Survey data collection and public reporting requirements — Continuation of the newness exception for FY 2023 and all subsequent years.
d. Survey participation requirements — Table 20 (reproduced below) restates the data submission dates for FY 2023 through FY 2025.
e. Proposal to add CAHPS Hospice Survey Star Ratings to public reporting — CMS proposes to introduce star ratings for public reporting of CAHPS Hospice Survey results on the Care Compare or successor websites no sooner than FY 2022 and proposes that the calculation and display of the CAHPS Hospice Survey Star Ratings be similar to that of other CAHPS Star Ratings programs such as Hospital CAHPS and Home Health CAHPS.
8. Form, manner, and timing of quality data submission — Hospices must comply with CMS submission data requirements. Table 22 (reproduced below) summarizes the HQRP compliance timeliness threshold required for a specific FY APU. CMS states that most hospices that fail to meet HQRP requirements do so because they miss the 90% threshold.
TABLE 22: HQRP Compliance Checklist

9. Public display of “quality measures” and other hospice data for HQRP — CMS proposes to publicly report the HVLDL and HCI, another claims-based measure, no earlier than May 2022. CMA also proposes, in the COVID-19 PHE, to use three quarters of HIS data of the final affected refresh, the February 2022 public reporting refresh of Care Compare for the Hospice setting.
Proposal for the January 2022 HH QRP public reporting display schedule with fewer than standard number of quarters due to COVID-19 PHE exemptions
This rule proposes changes to the HH QRP to report fewer quarters of data due to COVID-19 PHE.
Summary of impacts
The overall economic impact of this proposed rule is estimated to be $530 million in increased payments to hospices for FY 2022.
How we can help
Connect with CLA for further clarification on these proposed rules and how they impact hospice care providers. Our team of health care professionals is on the front lines of regulatory, policy, and payment changes for providers across the continuum and can provide guidance to meet your specific needs.
